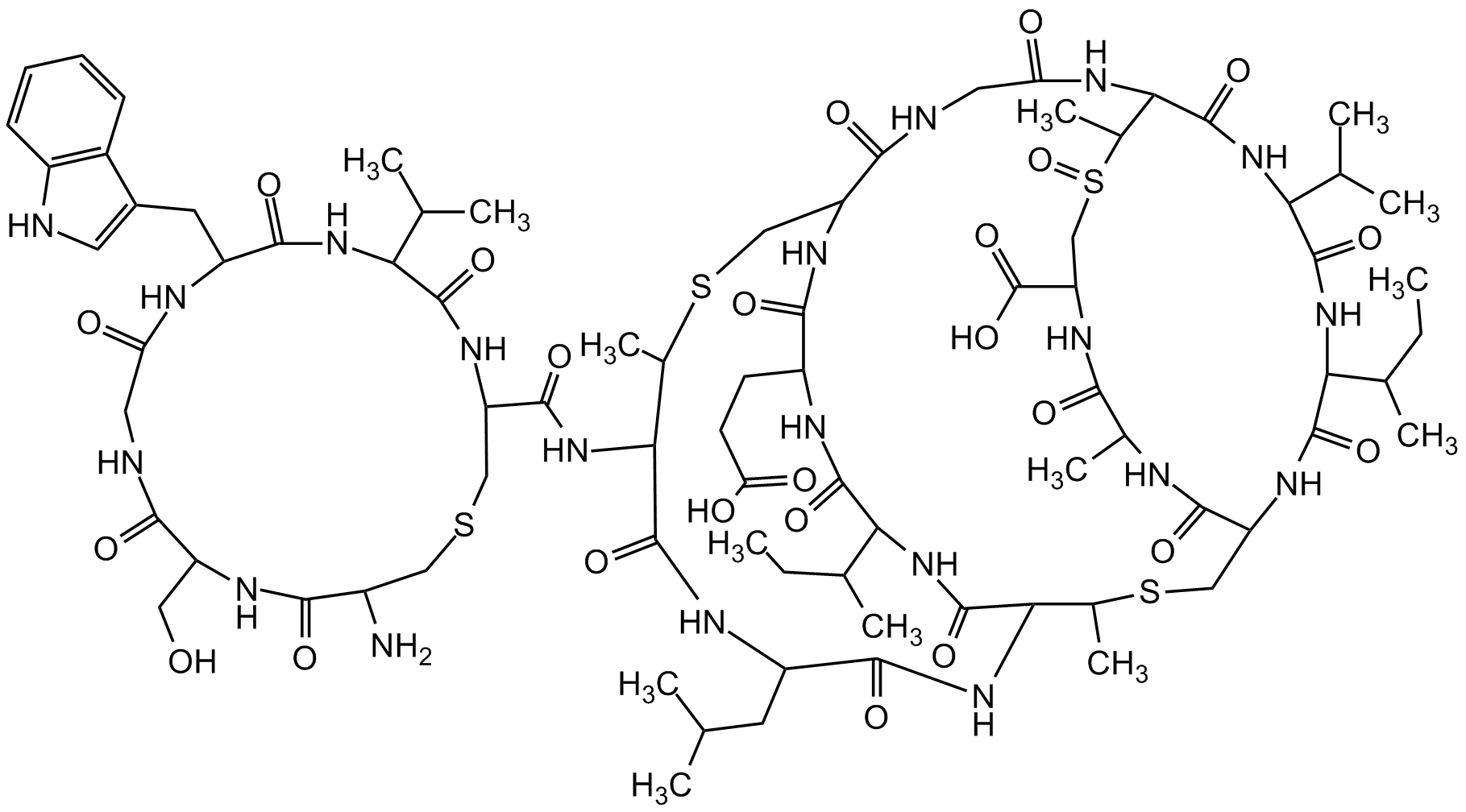
Actagardin
Product Code: AG-CN2-0300
Product Group: Natural Products and Extracts
Supplier: AdipoGen Life Sciences
| Code | Size | Price |
|---|
| AG-CN2-0300-M001 | 1 mg | £90.00 |
Quantity:
| AG-CN2-0300-M005 | 5 mg | £300.00 |
Quantity:
| AG-CN2-0300-M025 | 25 mg | £880.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Host Type: Bacteria
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
Gardimycin; Antibiotic A 3802-IV-3
Appearance:
Beige powder.
CAS:
59165-34-3
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C81H124N20O24S4/c1-15-37(9)60-76(118)88-47(21-22-57(105)106)69(111)91-52-31-127-40(12)62(101-73(115)51-30-126-29-45(82)66(108)90-50(28-102)67(109)84-26-55(103)87-49(71(113)96-58(35(5)6)74(116)92-51)24-43-25-83-46-20-18-17-19-44(43)46)78(120)89-48(23-34(3)4)70(112)100-63(79(121)99-60)41(13)128-32-53-72(114)86-39(11)65(107)94-54(81(123)124)33-129(125)42(14)64(95-56(104)27-85-68(52)110)80(122)97-59(36(7)8)75(117)98-61(38(10)16-2)77(119)93-53/h17-20,25,34-42,45,47-54,58-64,83,102H,15-16,21-24,26-33,82H2,1-14H3,(H,84,109)(H,85,110)(H,86,114)(H,87,103)(H,88,118)(H,89,120)(H,90,108)(H,91,111)(H,92,116)(H,93,119)(H,94,107)(H,95,104)(H,96,113)(H,97,122)(H,98,117)(H,99,121)(H,100,112)(H,101,115)(H,105,106)(H,123,124)
InChiKey:
LOHAAMWDKJNSML-UHFFFAOYSA-N
Long Description:
Chemical. CAS: 59165-34-3. Formula: C81H124N20O24S4. MW: 1890.2. Isolated from Actinoplanes sp. Tetracyclic class II lantibiotic. Structurally related to mersacidine. Antibiotic. Antibacterial against Gram-positive bacteria and Gram-negative microorganisms. Specifically inhibits peptidoglycan synthesis in the cell wall of Gram-positive bacteria, resulting in the accumulation of both uridine 5'-disphosphate-N-acetylmuramylpentapeptide and lipid II. Has the ability to form a complex with lipid intermediate II (lipid II).
MDL:
MFCD01772577
Molecular Formula:
C81H124N20O24S4
Molecular Weight:
1890.2
Package Type:
Vial
Product Description:
Tetracyclic class II lantibiotic. Structurally related to mersacidine. Antibiotic. Antibacterial against Gram-positive bacteria and Gram-negative microorganisms. Specifically inhibits peptidoglycan synthesis in the cell wall of Gram-positive bacteria, resulting in the accumulation of both uridine 5'-disphosphate-N-acetylmuramylpentapeptide and lipid II. Has the ability to form a complex with lipid intermediate II (lipid II).
Purity:
>80% (HPLC)
SMILES:
O=C(NC(C(C)C)C(NC1C(NC(C(NC(CC(C)C)C(NC(C(C)SCC2NC3=O)C(NC(C(C)CC)C(NC4CCC(O)=O)=O)=O)=O)=O)C(SCC(C(NCC(NC(C(S(CC(C(O)=O)NC(C(NC2=O)C)=O)=O)C)C(NC(C(C)C)C(NC3C(C)CC)=O)=O)=O)=O)NC4=O)C)=O)=O)C(NC(CNC(C(CO)NC(C(N)CSC1)=O)=O)=O)CC5=CNC6=C5C=CC=C6
Solubility Chemicals:
Soluble in ethanol, DMSO or aqueous acetonitrile.
Source / Host:
Isolated from Actinoplanes sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
References
Gardimycin, a new antibiotic from Actinoplanes. I. Description of the producer strain and fermentation studies: F. Parenti, et al.; J. Antibiot. 29, 501 (1976) | Gardimycin, a new antibiotic from Actinoplanes. II Isolation and preliminary characterisation: C. Coronelli, et al.; J. Antibiot. 29, 507 (1976) | The Three-Dimensional Solution Structure of the Lantibiotic Murein-Biosynthesis-Inhibitor Actagardine Determined by NMR: N. Zimmermann & G. Jung; Eur. J. Biochem. 246, 809 (1997) | New insights into the mechanism of action of lantibiotics - Diverse biological effects by binding to the same molecular target: H. Br?tz & H.G. Sahl; J. Antimicr. Chemother. 46, 1 (2000) | Lantibiotics produced by Actinobacteria and their potential applications (a review): K. Machado Gomes, et al.; Microbiol. 163, 109 (2017)
Related Products
| Product Name | Product Code | Supplier | NAI-857 | AG-CN2-0311 | AdipoGen Life Sciences | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAI-97 [Planosporicin] | AG-CN2-0312 | AdipoGen Life Sciences | Summary Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||