NAI-112
Product Code:
AG-CN2-0309
AG-CN2-0309
Host Type:
Bacteria
Bacteria
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0309-M001 | 1 mg | £85.00 |
Quantity:
| AG-CN2-0309-M005 | 5 mg | £310.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Lantibiotic NAI 112
Appearance:
Beige powder.
CAS:
1498289-64-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C109H149N25O32S2/c1-10-60-86(144)116-62(33-50(2)3)94(152)129-108(38-67(89(147)115-60)120-99(157)81(111)51(4)5)49-167-47-73(125-98(156)78-29-20-32-133(78)102(160)72(46-138)124-93(151)71(45-137)123-100(158)82(52(6)7)128-106(108)164)103(161)131-30-18-27-76(131)96(154)113-41-80(140)114-66(36-57-42-134(75-26-17-15-24-59(57)75)104-85(143)84(142)83(141)53(8)166-104)101(159)132-31-19-28-77(132)97(155)119-68-39-109(130-95(153)63(34-55-21-12-11-13-22-55)117-91(149)70(44-136)122-90(68)148)54(9)168-48-74(105(162)163)126-88(146)65(37-79(110)139)118-92(150)69(43-135)121-87(145)64(127-107(109)165)35-56-40-112-61-25-16-14-23-58(56)61/h10-17,21-26,40,42,50-54,62-74,76-78,81-85,104,112,135-138,141-143H,18-20,27-39,41,43-49,111H2,1-9H3,(H2,110,139)(H,113,154)(H,114,140)(H,115,147)(H,116,144)(H,117,149)(H,118,150)(H,119,155)(H,120,157)(H,121,145)(H,122,148)(H,123,158)(H,124,151)(H,125,156)(H,126,146)(H,127,165)(H,128,164)(H,129,152)(H,130,153)(H,162,163)/b60-10-
InChiKey:
PFZIDZPCKNACSM-QOJXDRFFSA-N
Long Description:
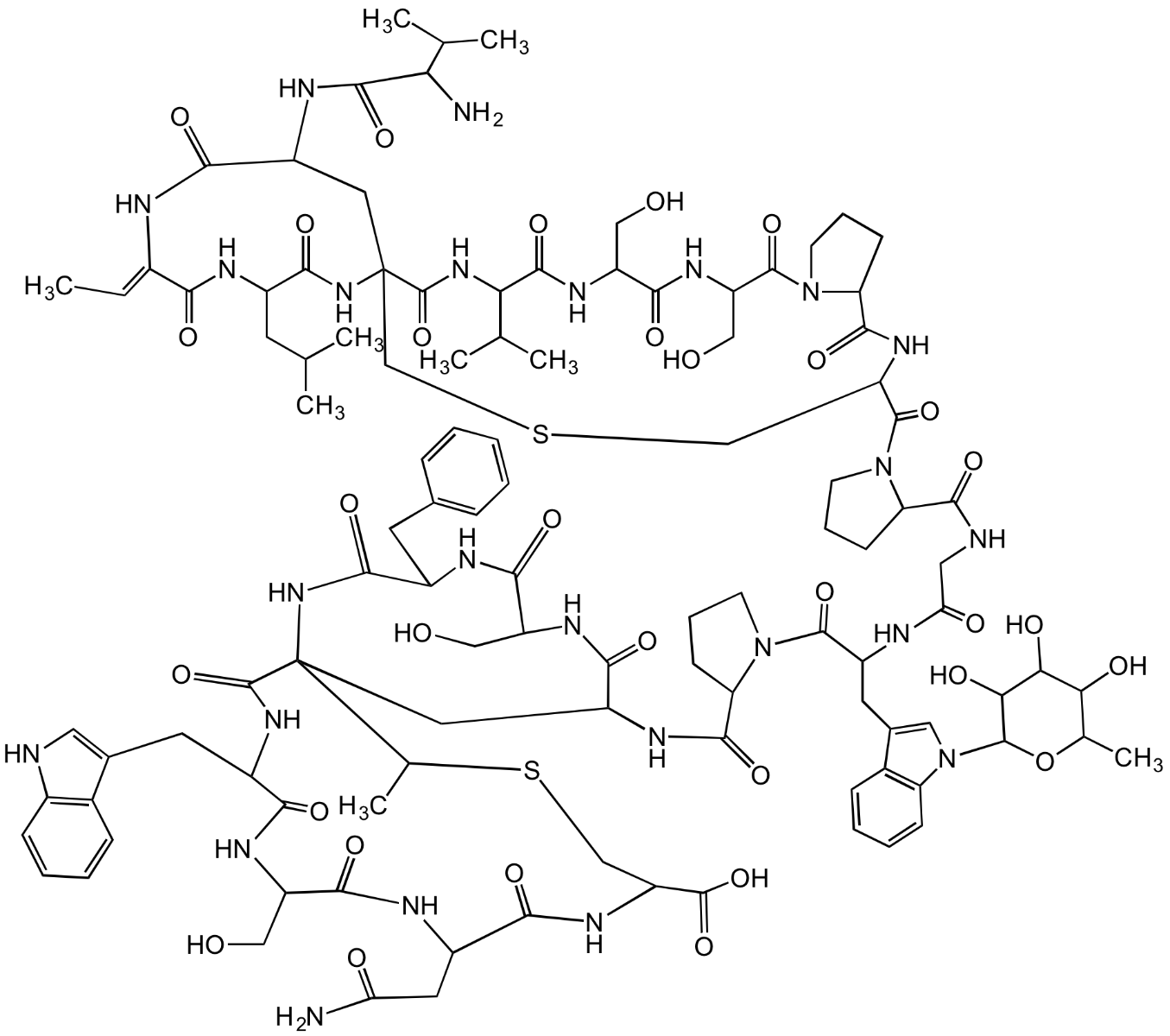
Chemical. CAS: 1498289-64-7. Formula: C109H147N25O31S2. MW: 2367.6. Isolated from Actinoplanes sp. Labionin-containing class III lanthipeptide. Antinociceptive agent. Effective in animal models of nociceptic and inflammatory pain. Does not act through opioid, cannabinoid or descending adrenergic mechanisms. Most likely acts through the vanilloid-sensitive pathway by a novel target. It can be developed for the treatment of neuropathic pain. Weak antibacterial compound.
Molecular Formula:
C109H147N25O31S2
Molecular Weight:
2367.6
Other data:
Purity Note: The purity is referred exclusively to the main congener(s), the sample also contains minor related congeners. See: A glycosylated, labionin-containing lanthipeptide with marked antinociceptive activity: M. Iorio, et al.; ACS Chem. Biol. 9, 398 (2014)
Package Type:
Vial
Product Description:
Labionin-containing class III lanthipeptide. Antinociceptive agent. Effective in animal models of nociceptic and inflammatory pain. Does not act through opioid, cannabinoid or descending adrenergic mechanisms. Most likely acts through the vanilloid-sensitive pathway by a novel target. It can be developed for the treatment of neuropathic pain. Weak antibacterial compound.
Purity:
>80% (HPLC)
SMILES:
O=C(NC(CC1(C(NC(CC2=CNC3=C2C=CC=C3)C(NC(CO)C(NC(CC(N)=O)C(NC(C(O)=O)CSC1C)=O)=O)=O)=O)NC4=O)C(NC(CO)C(NC4CC5=CC=CC=C5)=O)=O)C6CCCN6C(C(CC7=CN(C8OC(C)C(O)C(O)C8O)C9=C7C=CC=C9)NC(CNC(C%10N(C(C(CSC%11)NC(C%12N(CCC%12)C(C(CO)NC(C(CO)NC(C(C(C)C)NC(C%11%13NC(C(CC(C)C)NC(/C(NC(C(C%13)NC(C(C(C)C)N)=O)=O)=C/C)=O)=O)=O)=O)=O)=O)=O)=O)CCC%10)=O)=O)=O
Solubility Chemicals:
Soluble in DMSO, aqueous acetonitrile or ethanol.
Source / Host:
Isolated from Actinoplanes sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
A glycosylated, labionin-containing lanthipeptide with marked antinociceptive activity: M. Iorio, et al.; ACS Chem. Biol. 9, 398 (2014) | Discovering new bioactive molecules from microbial sources: P. Monciardini, et al.; Microb. Biotechnol. 7, 209 (2014) (Review)