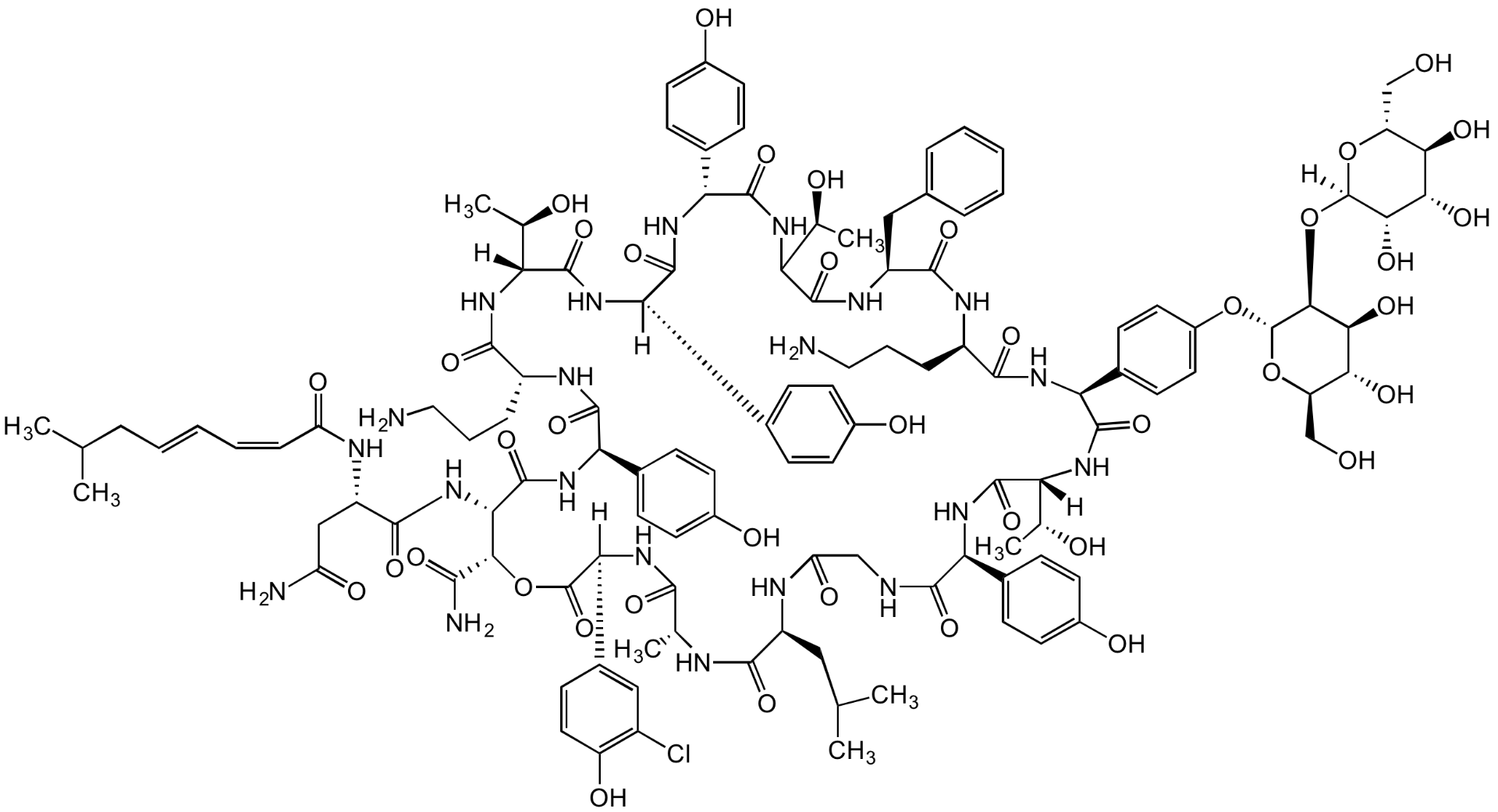
Ramoplanin A2
Product Code:
AG-CN2-0318
AG-CN2-0318
Host Type:
Bacteria
Bacteria
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| AG-CN2-0318-M001 | 1 mg | £45.00 |
Quantity:
| AG-CN2-0318-M005 | 5 mg | £115.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
Antibiotic A16686A2; MDL-62198; Antibiotic A 16686
Appearance:
White to off-white powder.
CAS:
81988-88-7 [A2]
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Keep cool and dry.Protect from light.Protect from light when in solution.
InChi:
InChI=1S/C119H154ClN21O40/c1-54(2)17-11-9-14-22-82(153)127-77(50-81(123)152)107(166)141-93-99(101(124)160)180-117(176)92(66-33-44-78(151)72(120)49-66)140-102(161)56(5)126-105(164)75(47-55(3)4)128-83(154)51-125-108(167)87(61-23-34-67(147)35-24-61)136-111(170)86(59(8)146)134-113(172)89(65-31-42-71(43-32-65)177-119-100(97(158)95(156)80(53-143)179-119)181-118-98(159)96(157)94(155)79(52-142)178-118)135-104(163)73(20-15-45-121)129-106(165)76(48-60-18-12-10-13-19-60)131-109(168)84(57(6)144)133-114(173)90(63-27-38-69(149)39-28-63)138-115(174)91(64-29-40-70(150)41-30-64)137-110(169)85(58(7)145)132-103(162)74(21-16-46-122)130-112(171)88(139-116(93)175)62-25-36-68(148)37-26-62/h9-14,18-19,22-44,49,54-59,73-77,79-80,84-100,118-119,142-151,155-159H,15-17,20-21,45-48,50-53,121-122H2,1-8H3,(H2,123,152)(H2,124,160)(H,125,167)(H,126,164)(H,127,153)(H,128,154)(H,129,165)(H,130,171)(H,131,168)(H,132,162)(H,133,173)(H,134,172)(H,135,163)(H,136,170)(H,137,169)(H,138,174)(H,139,175)(H,140,161)(H,141,166)/b11-9+,22-14-/t56-,57+,58-,59-,73-,74-,75+,76+,77+,79-,80-,84?,85-,86-,87+,88-,89+,90-,91+,92+,93+,94-,95-,96+,97+,98+,99+,100+,118-,119+/m1/s1
InChiKey:
KGZHFKDNSAEOJX-BALZYLSASA-N
Long Description:
Chemical. CAS: 81988-88-7 [A2]. Formula: C119H154ClN21O40. MW: 2554.1. Isolated from Actinoplanes sp. Glycolipodepsipeptide antibiotic. Complex of structurally related molecules A1, A2 and A3, with ramoplanin A2 as the primary component. Antibacterial and antiviral agent with activity against aerobic and anaerobic Gram-positive bacteria such as Clostridium difficile and antibiotic-resistant enterococci. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis.
MDL:
MFCD01775776
Molecular Formula:
C119H154ClN21O40
Molecular Weight:
2554.1
Other data:
Purity Note: The purity is referred exclusively to the main congener(s), the sample also contains minor related congeners. See: Ramoplanin (A-16686), a new glycolipodepsipeptide antibiotic. III. Structure elucidation: R. Ciabatti, et al.; J. Antibiot. 42, 254 (1989)
Package Type:
Vial
Product Description:
Glycolipodepsipeptide antibiotic. Complex of structurally related molecules A1, A2 and A3, with ramoplanin A2 as the primary component. Antibacterial and antiviral agent with activity against aerobic and anaerobic Gram-positive bacteria such as Clostridium difficile and antibiotic-resistant enterococci. Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis.
Purity:
>90% (HPLC)
SMILES:
OC[C@H]([C@@H](O)[C@H](O)[C@@H]1O)O[C@]1([H])O[C@@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)OC3=CC=C([C@@H]4NC([C@H](NC([C@@H](NC(C(NC([C@@H](C5=CC=C(O)C=C5)NC([C@@](C6=CC=C(O)C=C6)([H])NC([C@@](NC([C@H](NC([C@@H](C7=CC=C(O)C=C7)NC([C@@H](NC([C@H](CC(N)=O)NC(/C=CC=CCC(C)C)=O)=O)[C@@H](C(N)=O)OC([C@@](C8=CC(Cl)=C(O)C=C8)([H])NC([C@@H](C)NC([C@H](CC(C)C)NC(CNC([C@H](C9=CC=C(O)C=C9)NC([C@]([C@H](O)C)([H])NC4=O)=O)=O)=O)=O)=O)=O)=O)=O)CCCN)=O)([H])[C@H](O)C)=O)=O)=O)[C@@H](O)C)=O)CC%10=CC=CC=C%10)=O)CCCN)=O)C=C3
Solubility Chemicals:
Soluble in DMSO or water.
Source / Host:
Isolated from Actinoplanes sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
A-16686, a new antibiotic from Actinoplanes. I. Fermentation, isolation and preliminary physico-chemical characteristics: B. Cavalleri, et al.; J. Antibiot. 37, 309 (1984) | A-16686, a new antibiotic from Actinoplanes. II. Biological properties: R. Pallanza, et al.; J. Antibiot. 37, 318 (1984) | In vitro evaluation of ramoplanin (MDL 62198, A 16686): S. Dixson, et al.; Eur. J. Clin. Microbiol. Infect. Dis. 7, 819 (1988) | In vitro bactericidal activity of the glycopeptide compounds vancomycin, teicoplanin and ramoplanin (A-16686/MDL 62,198): M.D. O'Hare, et al.; J. Chemother. 1, 210 (1989) | In-vitro studies with ramoplanin (MDL 62,198): a novel lipoglycopeptide antimicrobial: M.D. O'Hare, et al.; J. Antimicrob. Chemother. 25, 217 (1990) | Inhibition of peptidoglycan biosynthesis by ramoplanin: E.A. Somner & P.E. Reynolds; Antimicrob. Agents Chemother. 34, 413 (1990) | In-vitro activity of vancomycin, teicoplanin, daptomycin, ramoplanin, MDL 62873 and other agents against staphylococci, enterococci and Clostridium difficile: A. Bartoloni, et al.; J. Antimicrob. Chemother. 26, 627 (1990) | Bactericidal activity of ramoplanin against antibiotic-resistant enterococci: C.C. Johnson, et al.; Antimicrob. Agents Chemother. 36, 2342 (1992) | In vitro activity of ramoplanin against vancomycin-resistant gram-positive organisms: L.A. Collins, et al.; Antimicrob. Agents Chemother. 37, 1364 (1993) | A new structure for the substrate-binding antibiotic ramoplanin: M.C. Lo, et al.; JACS 123, 8640 (2001) | Ramoplanin. A 16686, A 16686A, MDL 62198:Adis Comments; Drugs R.D. 3, 271 (2002) (Review) | Ramoplanin inhibits bacterial transglycosylases by binding as a dimer to lipid II: Y. Hu, et al.; JACS 125, 8736 (2003) | Chemistry and biology of ramoplanin: a lipoglycodepsipeptide with potent antibiotic activity: S. Walker, et al.; Chem. Rev. 105, 449 (2005) (Review) | Ramoplanin: a lipoglycodepsipeptide antibiotic: D.K. Farver, et al.; Ann. Pharmacother. 39, 863 (2005) (Review) | Lipid II as a target for antibiotics: E. Breukink & B. de Kruijff; Nat. Rev. Drug Discov. 5, 321 (2006) (Review) | The mechanism of action of ramoplanin and enduracidin: X. Fang, et al.; Mol. Biosyst. 2, 69 (2006) | Cyclic lipodepsipeptides: a new class of antibacterial agents in the battle against resistant bacteria: N. Bionda, et al.; Future Med. Chem. 5, 1311 (2013) (Review)