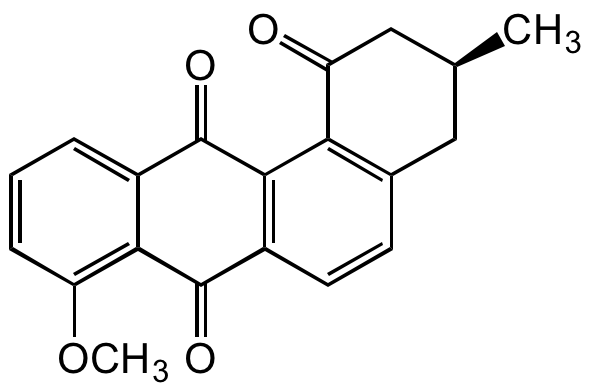
Rubiginone B2
| Code | Size | Price |
|---|
| BVT-0026-M001 | 1 mg | £155.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
-20°C
Images
Documents
Further Information
Alternate Names/Synonyms:
(+)-Rubiginone B2; X-14881C; 8-O-Methyl-ochramycinone
Appearance:
Yellow solid.
CAS:
130548-10-6
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS07,GHS08
Handling Advice:
Keep cool and dry.Protect from light when in solution.
Hazards:
H302, H312, H319, H351
InChi:
InChI=1S/C20H16O4/c1-10-8-11-6-7-13-18(16(11)14(21)9-10)20(23)12-4-3-5-15(24-2)17(12)19(13)22/h3-7,10H,8-9H2,1-2H3/t10-/m0/s1
InChiKey:
ZUCWNLVDTXGGSU-JTQLQIEISA-N
Long Description:
Chemical. CAS: 130548-10-6. Formula: C20H16O4. MW: 320.3. Isolated from Streptomyces sp. Angucyclinone antibiotic. Antibacterial and antitumor agent. Potentiates the cytotoxicity of vincristine. Antimalarial agent.
MDL:
MFCD00895768
Molecular Formula:
C20H16O4
Molecular Weight:
320.3
Package Type:
Plastic Vial
Precautions:
P201, P270, P281, P301, P312, P302, P352, P405
Product Description:
Angucyclinone antibiotic. Antibacterial and antitumor agent. Potentiates the cytotoxicity of vincristine. Antimalarial agent.
Purity:
>98% (HPLC)
Signal Word:
Warning
SMILES:
O=C1C2=C(C(C(C[C@@H](C)C3)=O)=C3C=C2)C(C4=CC=CC(OC)=C41)=O
Solubility Chemicals:
Soluble in DMSO, methanol, acetone or dichloromethane.
Source / Host:
Isolated from Streptomyces sp.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C.
References
Microbial products XI. Five novel metabolites related to benz[a]anthracene from an unidentified actinomycete designated X-14881: H. Maehr, et al.; J. Antibiot. 35, 1627 (1982) | Chemical and biological properties of rubiginone, a complex of new antibiotics with vincristine-cytotoxicity potentiating activity: M. Oka, et al.; J. Antibiot. 43, 967 (1990) | Absolute configuration of the rubiginones and photo-induced oxidation of the C1 hydroxyl of the antibiotics to a ketone: M. Oka, et al.; Tetrahed. Lett. 31, 7473 (1990) | Angucycline group antibiotics: J. Rohr & R. Thiericke; Nat. Prod. Rep. 9, 103, (1992) | New biologically active rubiginones from Streptomyces sp.: C. Puder, et al.; J. Antibiot. 53, 329 (2000) | Angucyclinone Antibiotics: Total Syntheses of YM-181741, (+)-Ochromycinone, (+)-Rubiginone B2, Tetrangomycin and MM-47755: K.P. Kaliappan & V. Ravikumar; J. Org. Chem. 72, 6116 (2007) | Saccharosporones A, B and C, cytotoxic antimalarial angucyclinones from Saccharopolyspora sp. BCC 21906: C. Boonlarppradab, et al.; J. Antibiot. 66, 305 (2013)