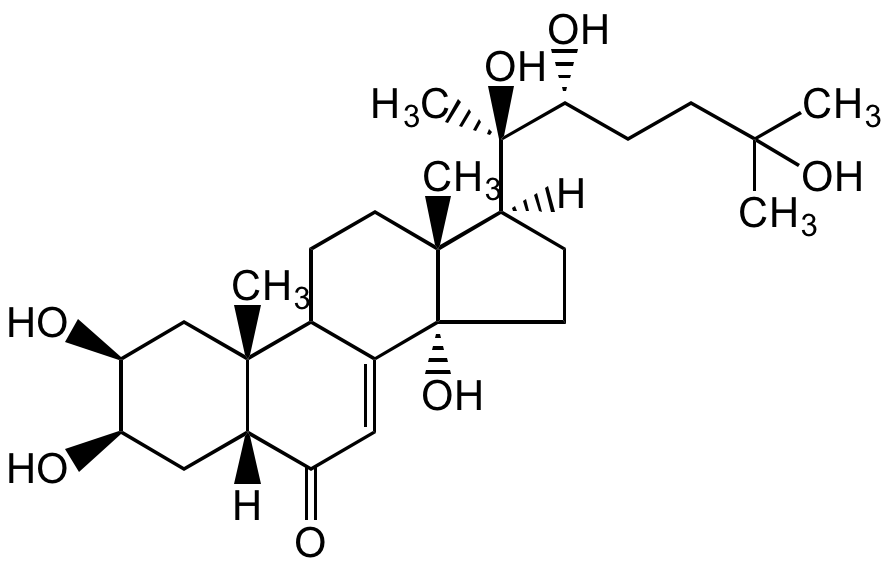
Ecdysterone
Product Code:
CDX-E0225
CDX-E0225
Regulatory Status:
RUO
RUO
Shipping:
AMBIENT
AMBIENT
Storage:
-20°C
-20°C
No additional charges, what you see is what you pay! *
| Code | Size | Price |
|---|
| CDX-E0225-M005 | 5 mg | £96.00 |
Quantity:
| CDX-E0225-M025 | 25 mg | £267.00 |
Quantity:
Prices exclude any Taxes / VAT
Stay in control of your spending. These prices have no additional charges, not even shipping!
* Rare exceptions are clearly labelled (only 0.14% of items!).
* Rare exceptions are clearly labelled (only 0.14% of items!).
Multibuy discounts available! Contact us to find what you can save.
This product comes from: Switzerland.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
Typical lead time: 7-10 working days.
Contact us for more accurate information.
- Further Information
- Documents
- References
- Related Products
- Show All
Further Information
Alternate Names/Synonyms:
20-Hydroxyecdysone; beta-Ecdysone; 2beta,3beta,14alpha,20beta,22,25-Hexahydroxy-7-cholesten-6-one; Insect moulting hormone; Polypodine A
Appearance:
White to off-white powder.
CAS:
5289-74-7
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C27H44O7/c1-23(2,32)9-8-22(31)26(5,33)21-7-11-27(34)16-12-18(28)17-13-19(29)20(30)14-24(17,3)15(16)6-10-25(21,27)4/h12,15,17,19-22,29-34H,6-11,13-14H2,1-5H3/t15?,17-,19+,20-,21-,22+,24+,25+,26+,27+/m0/s1
InChiKey:
NKDFYOWSKOHCCO-MQMWGCQISA-N
Long Description:
Chemical. CAS: 5289-74-7. Formula: C27H44O7. MW: 480.63. Synthetic. A member of the ecdysteroid family. Ecdysone receptor (EcR) agonist. More potent than ecdysone. Induces the expression of genes coding for proteins that the larva requires, and it causes chromosome puffs (sites of high expression) to form in polytene chromosomes. Plays a key role in insect development, cell proliferaton, growth and apoptosis by controlling gene expression involved in moulting and metamorphosis. It acts through a heterodimeric receptor comprising the ecdysone receptor and the ultraspiracle proteins (USP). Used for controlled gene expression in scientific research, agriculture and medicine. Used for the development of selective insect growth regulators for use as environmentally benign insecticides. Shows biological effects on mammalian species, including antiepileptic, antidiabetic, antiobesity, ROS-inhibiting activity.ht produces vitamin D3. UV/Vis: lambdamax 271, 282, 293nm.
MDL:
MFCD00036740
Molecular Formula:
C27H44O7
Molecular Weight:
480.63
Package Type:
Vial
Product Description:
A member of the ecdysteroid family. Ecdysone receptor (EcR) agonist. More potent than ecdysone. Induces the expression of genes coding for proteins that the larva requires, and it causes chromosome puffs (sites of high expression) to form in polytene chromosomes. Plays a key role in insect development, cell proliferaton, growth and apoptosis by controlling gene expression involved in moulting and metamorphosis. It acts through a heterodimeric receptor comprising the ecdysone receptor and the ultraspiracle proteins (USP). Used for controlled gene expression in scientific research, agriculture and medicine. Used for the development of selective insect growth regulators for use as environmentally benign insecticides. Shows biological effects on mammalian species, including antiepileptic, antidiabetic, antiobesity, ROS-inhibiting activity.ht produces vitamin D3. UV/Vis: lambdamax 271, 282, 293nm.
Purity:
>97% (HPLC)
SMILES:
[H][C@@]1(CC[C@@]2(O)C3=CC(=O)[C@]4([H])C[C@@H](O)[C@@H](O)C[C@]4(C)C3CC[C@]12C)[C@@](C)(O)[C@H](O)CCC(C)(C)O
Solubility Chemicals:
Soluble in methanol (20mg/ml), DMSO or ethanol.
Source / Host:
Synthetic
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at -20°C.
Documents
References
(1) F. Engelmann; Science 174, 1041 (1971) | (2) S. Tsujiyama, et al.; Jpn. J. Pharmacol. 68, 133 (1995) | (3) E.H. Baehrecke, et al.; Arch. Insect Biochem. Physiol. 33, 231 (1996) | (4) R. Hanaya, et al.; Jpn. J. Pharmacol. 74, 331 (1997) | (5) L.M. Riddiford, et al.; Vitam. Horm. 60, 1 (2000) | (6) C.S Thummel; Insect Biochem. Mol. Biol. 32, 113 (2002) | (7) L.D. Graham; Expert Opin. Biol. Ther. 2, 525 (2002) | (8) P. Kizelsztein, et al.; Am. J. Physiol. Endocrinol. Metab. 296, E433 (2009) | (9) J. Hu, et al.; J. Cell Biochem. 111, 1512 (2010)
Related Products
| Product Name | Product Code | Supplier | 7beta-Hydroxy-cholesteryl-bishemisuccinate-diethanolamine salt | CDX-H0123 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|