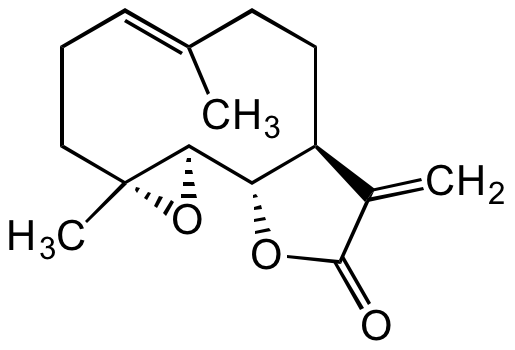
Parthenolide
| Code | Size | Price |
|---|
| CDX-P0297-M025 | 25 mg | £72.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
2,3,6,7,7aS,8,10aS,10bR-octahydro-1aR,5-dimethyl-8-methylene-(4E)-oxireno[9,10]cyclodeca[1,2-b]furan-9(1aH)-one
Appearance:
White to off-white powder.
CAS:
20554-84-1
EClass:
32160000
Form (Short):
solid
GHS Symbol:
GHS08
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C15H20O3/c1-9-5-4-8-15(3)13(18-15)12-11(7-6-9)10(2)14(16)17-12/h5,11-13H,2,4,6-8H2,1,3H3/b9-5+/t11-,12-,13+,15+/m0/s1
InChiKey:
KTEXNACQROZXEV-PVLRGYAZSA-N
Long Description:
Chemical. CAS: 20554-84-1. Formula: C15H20O3. MW: 248.32. Synthetic. Anticancer and antiangiogenic compound. Induces apoptosis, cell cycle arrest and autophagy in various cancer cell lines. At low concentrations acts as antioxidant reducing oxidative stress generated through the TCR signaling pathway. At high concentration induces O2- and causes oxidative-stress-mediated apoptosis. Potent anti-inflammatory and anti-atherosclerotic agent. NF-kappaB inhibitor by directly targeting IkappaB kinase. NLRP3 inflammasome inhibitor. Inhibits ATPase activity of NLRP3 and protease activity of caspase 1. Specifically inhibits HDAC1 without affecting other class I/II HDACs. DNA methyltransferase 1 (DNMT1) inhibitor. Reduces the expression of VEGF and its receptors VEGRF1 and 2. Microtubule-interfering compound, inhibiting cell migration and tubule formation. Anti-leishmanial and anti-trypanosomal agent.
MDL:
MFCD00134592
Molecular Formula:
C15H20O3
Molecular Weight:
248.32
Package Type:
Vial
Product Description:
Anticancer and antiangiogenic compound. Induces apoptosis, cell cycle arrest and autophagy in various cancer cell lines. At low concentrations acts as antioxidant reducing oxidative stress generated through the TCR signaling pathway. At high concentration induces O2- and causes oxidative-stress-mediated apoptosis. Potent anti-inflammatory and anti-atherosclerotic agent. NF-kappaB inhibitor by directly targeting IkappaB kinase. NLRP3 inflammasome inhibitor. Inhibits ATPase activity of NLRP3 and protease activity of caspase 1. Specifically inhibits HDAC1 without affecting other class I/II HDACs. DNA methyltransferase 1 (DNMT1) inhibitor. Reduces the expression of VEGF and its receptors VEGRF1 and 2. Microtubule-interfering compound, inhibiting cell migration and tubule formation. Anti-leishmanial and anti-trypanosomal agent.
Purity:
>98% (HPLC)
SMILES:
C/C1=CCC[C@](O2)(C)[C@H]2[C@@H](OC(C3=C)=O)[C@H]3CC1
Solubility Chemicals:
Soluble in DMSO, ethanol or chloroform.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) R.M. Wiedhopf, et al.; J. Pharm. Sci. 62, 345 (1973) | (2) B.H. Kwok, et al.; Chem. Biol. 8, 759 (2001) | (3) P. Pozarowski, et al.; Cell Cycle 2, 377 (2003) | (4) T.S. Tiuman, et al.; Antimicrob. Agents Chemother. 49, 176 (2005) | (5) M. Li-Weber, et al.; Cell Death Differ. 12, 408 (2005) | (6) Y.N. Gopal, et al.; Chem. Biol. 14, 813 (2007) | (7) E. Izumi, et al.; Exp. Parasitol. 118, 324 (2008) | (8) Z. Liu, et al.; J. Pharmacol. Exp. Ther. 329, 505 (2009) | (9) C. Juliana, et al.; J. Biol. Chem. 285, 9792 (2010) | (10) L. Zhao, et al.; Nutr. Rev. 69, 310 (2011) (Review) | (11) V.B. Mathema, et al.; Inflammation 35, 560 (2012) (Review) | (12) S.L. Kim, et al.; Int. J. Mol. Med. 33, 1261 (2014)