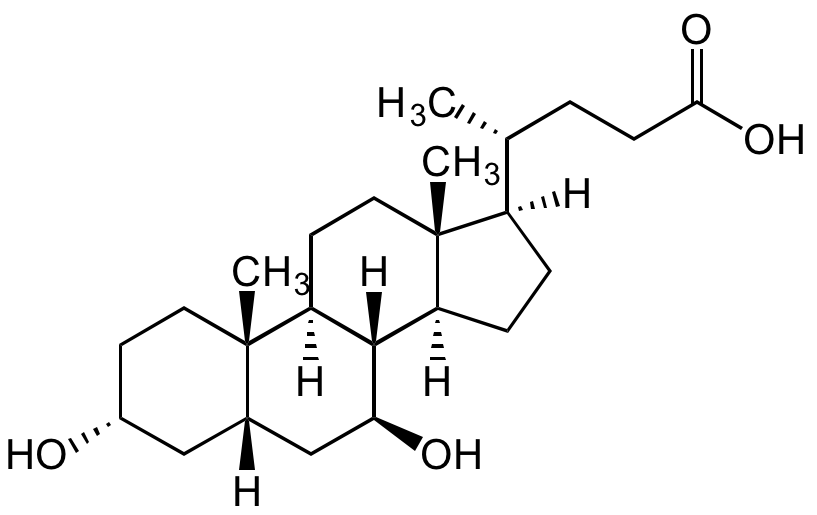
Ursodeoxycholic acid
| Code | Size | Price |
|---|
| CDX-U0019-G005 | 5 g | £121.00 |
Quantity:
| CDX-U0019-G025 | 25 g | £450.00 |
Quantity:
Prices exclude any Taxes / VAT
Overview
Regulatory Status: RUO
Shipping:
AMBIENT
Storage:
+4°C
Images
Documents
Further Information
Alternate Names/Synonyms:
UDCA; USAN; BRN 3219888; NSC 657950; NSC 683769; 3alpha,7beta-Dihydroxy-5beta-cholan-24-oic acid; 5beta-Cholan-24-oic acid-3alpha,7beta-diol; 7beta-Hydroxylithocholic acid; UDCS
Appearance:
White to off-white powder.
CAS:
128-13-2
EClass:
32160000
Form (Short):
liquid
Handling Advice:
Protect from light and moisture.
InChi:
InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1
InChiKey:
RUDATBOHQWOJDD-UZVSRGJWSA-N
Long Description:
Chemical. CAS: 128-13-2. Formula: C24H40O4. MW: 392.57. Synthetic. Endogenous hydrophilic bile acid. Antioxidant. Cytoprotective against oxidative stress and cell death. Hepatoprotective at cellular and molecular level, including stabilization of membranes. Antiapoptotic and antinecrotic. Targets the mitochondrial function and integrity, reduction of endoplasmatic stress and interactions with survival signals in cAMP, Akt, NF-kappaB, MAPK and PI3K signaling pathways. Chemopreventive against colorectal cancer by countering the tumor-promoting effects of secondary bile acids. Shows also effects on epidermal growth factor receptor (EGFR) signaling and COX-2 expression. Immunomodulator and anti-inflammatory compound. Modifies TLR4 and TLR9 signaling pathways and downregulates the production of proinflammatory tumor necrosis factor-alpha (TNF-alpha). Pregnane X receptor agonist. Neuroprotective. Anticholestatic agent. Used to reduce cholesterol absorption and for cholesterol gallstone dissolution.
MDL:
MFCD00003680
Molecular Formula:
C24H40O4
Molecular Weight:
392.57
Package Type:
Vial
Product Description:
Endogenous hydrophilic bile acid. Antioxidant. Cytoprotective against oxidative stress and cell death. Hepatoprotective at cellular and molecular level, including stabilization of membranes. Antiapoptotic and antinecrotic. Targets the mitochondrial function and integrity, reduction of endoplasmatic stress and interactions with survival signals in cAMP, Akt, NF-kappaB, MAPK and PI3K signaling pathways. Chemopreventive against colorectal cancer by countering the tumor-promoting effects of secondary bile acids. Shows also effects on epidermal growth factor receptor (EGFR) signaling and COX-2 expression. Immunomodulator and anti-inflammatory compound. Modifies TLR4 and TLR9 signaling pathways and downregulates the production of proinflammatory tumor necrosis factor-alpha (TNF-alpha). Pregnane X receptor agonist. Neuroprotective. Anticholestatic agent. Used to reduce cholesterol absorption and for cholesterol gallstone dissolution.
Purity:
>99% (T)
SMILES:
[H][C@]1([C@@H](CCC(O)=O)C)CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@@]21C
Solubility Chemicals:
Soluble in DMSO or ethanol.
Source / Host:
Synthetic.
Transportation:
Non-hazardous
UNSPSC Category:
Natural Products/Extracts
UNSPSC Number:
12352200
Use & Stability:
Stable for at least 2 years after receipt when stored at +4°C.
References
(1) D. Lapenna, et al.; Biochem. Pharmacol. 64, 1661 (2002) (Review) | (2) G. Paumgartner, et al.; Hepatology 36, 525 (2002) (Review) | (3) T.C. Schreuder, et al.; World J. Gastroenterol. 14, 2474 (2008) (Review) | (4) J.D. Amaral, et al.; Trends Mol. Med. 15, 531 (2009) (Review) | (5) J.D. Amaral, et al.; J. Lipid. Res. 50, 1721 (2009) (Review) | (6) M. Trauner, et al.; Dig. Dis. 28, 220 (2010) (Review) | (7) J.D. Amaral, et al.; Curr. Pharm. Des. 16, 2493 (2010) (Review) | (8) L. Serfaty, et al.; Gastroenterol. Clin. Biol. 34, 516 (2010) (Review) | (9) M.G. Roma, et al.; Clin. Sci. (Lond). 121, 523 (2011) (Review)
Related Products
| Product Name | Product Code | Supplier | Sodium glycochenodeoxycholate | CDX-G0033 | Chemodex | Summary Details | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|